Virtual Pooled Registry Cancer Linkage System/BRANY Central IRB
BRANY IRB is contracted with National Cancer Institute (NCI) to serve as a Central IRB to review Virtual Pooled Registry – Cancer Linkage System (VPR–CLS) studies. The Common Rule requires use of a Central (or single) IRB for cooperative research. Reliance on a Central IRB can eliminate redundancy and provide a consistent, high quality, expert review process that protects privacy and confidentiality while saving time and resources.
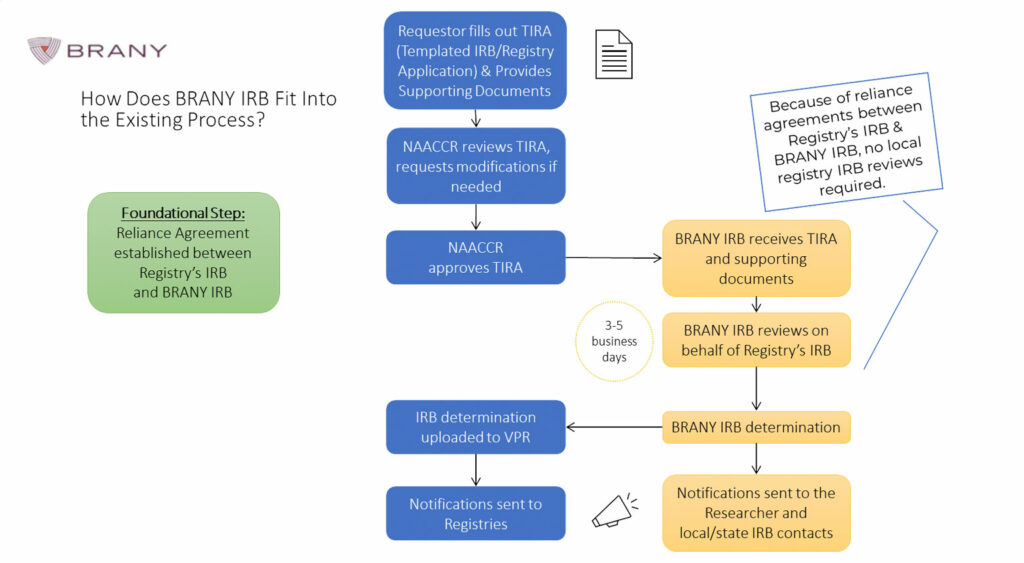
To streamline the Central IRB application and review process for VPR-CLS studies, the North American Association of Central Cancer Registries (NAACCR) has developed a Templated IRB/Registry Application (TIRA -common form). BRANY IRB will perform their review using the TIRA and other supporting documents submitted with the VPR-CLS request.
The flow diagram below outlines the process for submitting to NAACCR and BRANY IRB.
VPR-CLS Central IRB Submission Guidelines
Recent Presentations
- BRANY IRB as Central IRB for Review of VPR-CLS Linkage Requests: Purpose, Progress and Plans for 2022
- Streamlining the IRB Process
Request access to BRANY’s IRBManager
- Request for User Access - After you receive your initial approval, and would like access to IRBManager, please send a completed Request for User Access Form by email to Laura Donohue (or call 516-622-2049)
- IRBManager™ Demonstration Webinar
- Check the box to request a demonstration on the Request for User Access form
- Email rhart@brany.com
IRB Membership List — Compliance Statement — Meeting Schedule
- IRB Membership List (Current)
- BRANY IRB Calendar
- 2022 BRANY IRB Compliance Statement
- BRANY IRB Registration
BRANY IRB is registered per 45 CFR 46 Subpart E and 21 CFR 56.106 (Registration #IRB00000080 and #IRB00010793)
Reporting Timelines
Reporting Timelines
| UPIRTSOS (Unanticipated Problems) | Report per occurrence within 5 days |
| Serious Adverse Events (Local) | Report per occurrence within 5 days |
| Unanticipated Adverse Device Events (UADEs) | Report per occurrence within 10 days |
| Complaints | Report per occurrence within 5 days |
| Major Deviations | Report per occurrence within 10 days |
| Minor Deviations | Report in aggregate with continuing review or study closure |
